
Breadcrumb
- News and Events
- News
- Content
- New research aims to make prostate cancer prognostics more precise
null New research aims to make prostate cancer prognostics more precise
A team of RI-MUHC researchers is developing DNA biomarkers to better pinpoint treatment options for patients with prostate cancer
SOURCE: RI-MUHC
Prostate cancer is frequently diagnosed, accounting for an estimated 20% of new cancers in Canadian men in 2022. Distinguishing between slow-growing, indolent tumours and the more aggressive, potentially life-threatening form of prostate cancer is difficult. A team of researchers from the Research Institute of the McGill University Health Centre (RI-MUHC) set out to discover whether DNA-based biomarkers could add prognostic information to existing tools to better tailor treatment selection.
In a recent paper published in the British Journal of Cancer, a team led by Jacques Lapointe, MD, PhD, a researcher in the Cancer Research Program at the RI-MUHC, studied DNA copy number alteration (CNA) classifiers. Accurate assessment of these biomarkers has the potential to improve outcomes for prostate cancer patients with low or intermediate risk disease, but current assessment methods are cumbersome and costly. In this new study, the research team developed and validated an accessible tool that successfully predicted post-treatment tumour recurrence.
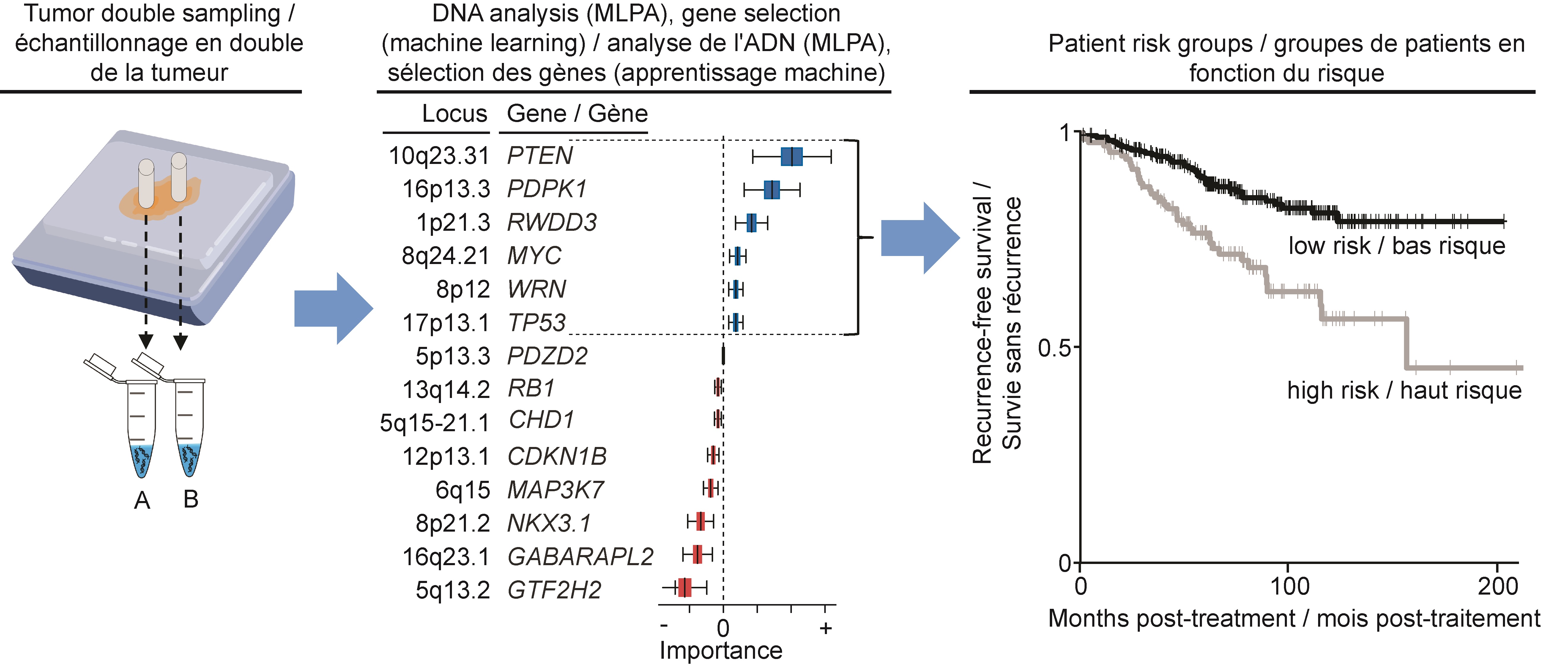
Working with 448 tumour samples from patients treated by prostatectomy at the McGill University Health Centre and at the Kingston General Hospital, Dr. Lapointe and his team initially identified 14 CNAs as potential biomarkers to predict prostate cancer recurrence.
“We assessed the CNA classifier with an inexpensive PCR-based assay called multiplex ligation-dependent probe amplification, or MLPA,” says Walead Ebrahimizadeh, first author of the study and a doctoral student in Dr. Lapointe’s lab at the time of this work. “We recently developed and optimized this assay, which detects gains and deletions in specific prostate cancer-relevant genes.”
MLPA provides a fast, easy and cost-effective approach for the assessment of candidate CNAs in small quantities of DNA. The team developed the MLPA assay to allow the assessment of all 14 selected CNAs at the same time.
“Previous research in our lab suggested that a combination of CNAs would help better stratify prostate cancer patients according to their risk of disease recurrence,” says Karl-Philippe Guérard, M.Sc., second author on the paper and a research associate in Dr. Lapointe’s laboratory.
“A unique aspect of our study was that we double-sampled in order to capture CNAs in low and high grade tumour areas,” adds Karl-Philippe Guérard. “This increases our predictive accuracy by accounting for the variability within a single tumour.”
The performance of the MLPA assay was confirmed using the “gold standard” methodology, fluorescence in situ hybridization.
“Our classifier marks a step toward personalized medicine for low or intermediate risk prostate cancers,” concludes Dr. Lapointe. “In the future, we hope to integrate CNA classifiers into routine clinical settings. Until then, our next step is to apply our MLPA assay to needle biopsies performed prior to prostate surgery and evaluate how the classifier can potentially assist decision-making when it comes to treatment.”
Read the publication in British Journal of Cancer
July 18, 2023
Related news
RI-MUHC study highlights the role of fat metabolism enzyme in aggressive prostate cancer