
Breadcrumb
- News and Events
- News
- Content
- RI-MUHC researchers identify new autophagy-regulating gene
null RI-MUHC researchers identify new autophagy-regulating gene
Researchers report how they were able to identify a previously unknown gene involved in regulating autophagy and skeletal muscle integrity
Source: RI-MUHC
Autophagy is a cellular process that plays a critical role in maintaining cellular homoeostasis by eliminating damaged or unnecessary components, such as misfolded proteins and organelles. This process plays an important role in maintaining cellular homeostasis and preventing the accumulation of toxic waste products within the cell. Autophagy is also a critical process in the regulation of muscle mass, function and integrity, but the molecular mechanisms behind it are complex and not yet fully understood.
In a new study published in Nature Communications and titled “MYTHO is a novel regulator of skeletal muscle autophagy and integrity,” researchers from the Research Institute of the McGill University Health Centre (RI-MUHC), Université du Québec à Montréal (UQÀM) and the University of Padova (UNIPD) report how they were able to identify a previously unknown gene involved in regulating autophagy and skeletal muscle integrity.

Autophagy is involved in a variety of physiological and pathological processes as a response to stress. For example, autophagy is important in maintaining muscle homoeostasis while exercising. However, defects in autophagy are also involved in a wide range of clinical conditions, including cancer, neurodegenerative disorders, muscular dystrophy, sepsis, multiple sclerosis and aging. A better understanding of autophagy could lead to better understanding of various methods of treatment for these diseases. This particular study, led by Dr. Sabah Hussain, of the RI-MUHC’s Translational Research in Respiratory Diseases Program, focused on a novel gene that regulates skeletal muscle integrity. Dr. Hussain is a distinguished James McGill Professor of Medicine who is studying the molecular mechanisms that are involved in skeletal muscle atrophy.
Through a series of innovative experiments and analyses, the team of scientists was able to identify and characterize a novel FoxO-dependent gene, d230025d16rik, which they named Mytho (Macroautophagy and YouTH Optimizer). They discovered that MYTHO is a regulator of autophagy and skeletal muscle integrity in vivo.
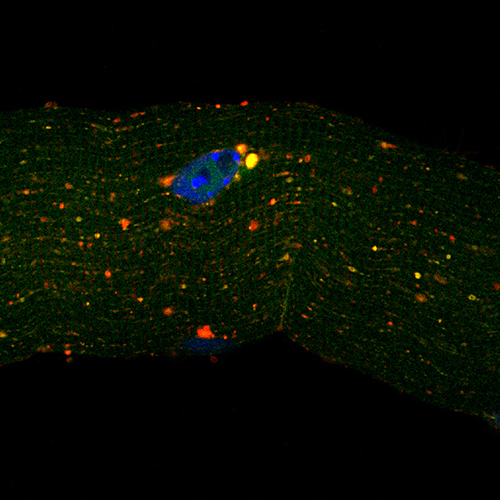
The team of scientists found that Mytho is significantly up-regulated in various mouse models of skeletal muscle atrophy. The researchers then showed that short-term depletion of Mytho in mice attenuates muscle atrophy caused by fasting, denervation, cancer cachexia and sepsis.
In a series of subsequent experiments, researchers also found that prolonged Mytho depletion is associated with severe muscular disorders, impaired autophagy, muscle weakness, degeneration of muscle tissue and extensive ultrastructural defects.
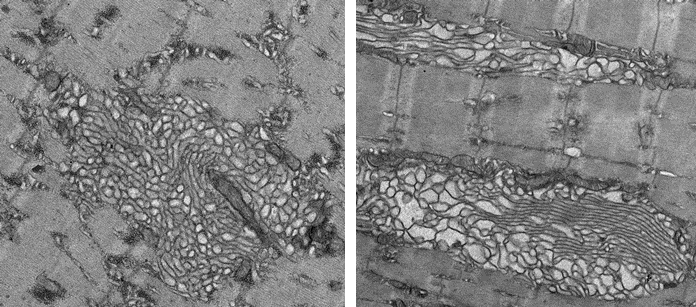
The researchers then found that inhibition of the mTORC1 signaling pathway in mice using rapamycin treatment attenuates the myopathic phenotype triggered by MYTHO knockdown. In other experiments, it was discovered that human patients diagnosed with myotonic dystrophy type 1 (DM1) display reduced Mytho expression, activation of the mTORC1 signaling pathway and impaired autophagy. This suggests that dysregulation of Mytho expression may also contribute to human muscular diseases.
"This discovery is a significant breakthrough in our understanding of the regulation of autophagy and skeletal muscle integrity. The gene we have identified plays a crucial role in skeletal muscle atrophy and could provide a new target for the development of therapies for muscle-related diseases."
— Lead authors Jean-Philippe Leduc-Gaudet, PhD, and Anais Franco-Romero, PhD

About the study
Read the publication in Nature Communications.
The study was funded by the Canadian Institutes of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC), and Fonds de recherche du Québec - Santé (FRQS), and was supported by the AFM-Téléthon.
April 28, 2023