
Fil d'Ariane
- Nouvelles et événements
- Nouvelles
- Content
- Une équipe de l’IR-CUSM étudie un nouveau système d’administration d’insuline, entièrement automatisé
null Une équipe de l’IR-CUSM étudie un nouveau système d’administration d’insuline, entièrement automatisé
Une nouvelle publication dans Lancet Digital Health décrit une percée scientifique majeure, susceptible d’améliorer le contrôle de la glycémie et la qualité de vie des personnes vivant avec le diabète de type 1
SOURCE : IR-CUSM. À l’approche de la Journée mondiale du diabète, le 14 novembre prochain, une équipe de l’Institut de recherche du Centre universitaire de santé McGill (IR-CUSM) rapporte que son étude récemment publiée dans Lancet Digital Health pourrait se traduire par une amélioration du contrôle de la glycémie et de la qualité de vie des personnes vivant avec le diabète de type 1.
Le diabète de type 1 est une maladie chronique; chez les personnes atteintes de cette affection, le pancréas ne peut pas produire adéquatement l’insuline nécessaire aux fonctions du corps. Les personnes vivant avec le diabète de type 1 doivent prendre leur vie durant de l’insuline de remplacement, en s’injectant quotidiennement de l’insuline ou en utilisant une pompe à insuline. Les percées technologiques ont entraîné la mise au point de systèmes automatisés d’administration d’insuline, appelés système d’administration d’insuline en boucle fermée ou système de pancréas artificiel.
« Les systèmes de pancréas artificiel réduisent les variations des taux de sucre et améliorent le contrôle de la glycémie comparativement aux pompes à insuline standards, déclare Ahmad Haidar, Ph. D., scientifique au Programme de recherche en désordres métaboliques et leurs complications à l’IR-CUSM et auteur principal de l’étude. Toutefois, les utilisateurs d’un système de pancréas artificiel doivent encore compter et noter la teneur en glucides de leurs repas, ce qui constitue un fardeau et une source d’erreurs. Notre objectif est d’alléger ce fardeau, grâce à la mise au point d’un nouveau système de pancréas artificiel entièrement automatisé, qui n’oblige pas à tenir compte de ce qui a été consommé aux repas. »

« On estime à quelque 20 % le pourcentage d’erreur d’estimation lorsque le calcul et l’enregistrement de la teneur en glucides de tous les repas se font manuellement; ces obligations ont également une incidence défavorable sur la qualité de vie des personnes vivant avec le diabète de type 1, plus particulièrement dans leurs interactions avec des pairs impliquant de la nourriture », ajoute la copremière auteure de l’étude, Dorsa Majdpour, M. Ing., actuellement étudiante en médecine à l’Université McGill.
Le traitement optimal du diabète de type 1 est un système de pancréas artificiel entièrement automatisé, qui allège le lourd fardeau que représente la nécessité de compter et de noter la teneur en glucides des aliments consommés, sans dégrader le contrôle de la glycémie.
« Nous avons réalisé le premier essai clinique randomisé visant à évaluer l’efficacité d’un système de pancréas artificiel de cette nature, n’imposant pas à l’utilisateur l’obligation de compter la teneur en glucides de tous les aliments, poursuit le Dr Michael Tsoukas, chercheur principal de l’essai clinique et copremier auteur de la publication. Nous avons comparé le système en boucle fermée Fiasp plus pramlintide de Novo Nordisk, sans prise en compte de ce qui avait été consommé lors des repas, avec le système Fiasp en boucle fermée seul, nécessitant le calcul précis des glucides; le premier est un système de pancréas artificiel entièrement automatisé, et le second est un système hybride. »
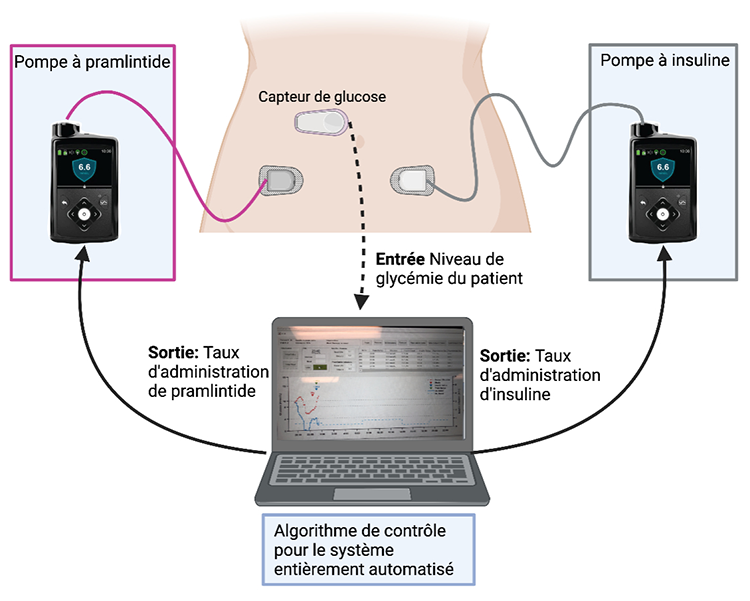
L’équipe a testé deux systèmes de pancréas artificiel lors d’un essai clinique réalisé au Centre universitaire de santé McGill, à Montréal, auprès de 24 participants en milieu hospitalier. Une équipe multidisciplinaire, formée d’ingénieurs, d’infirmières, de médecins et de membres du personnel de recherche, a réalisé l’essai clinique au cours de la période comprise entre le 8 février 2019 et le 19 septembre 2020.
« L’un des aspects les plus enthousiasmants du projet a été de constater l’engagement et l’intérêt des participants, commente Dorsa Majdpour. Des personnes de l’ensemble des États-Unis et du Canada ont communiqué avec nous, afin de nous manifester leur intérêt à participer à notre étude. »
Les chercheurs ont observé une hyperglycémie transitoire au cours des deux premières heures suivant les repas; toutefois, le système entièrement automatisé a toujours atteint un pourcentage de temps passé dans la cible (3,9 à 10,0 mmol/l) élevé quant au contrôle de la glycémie, et ce, sans événements indésirables.
« Nos résultats démontrent que les études sur le système en boucle fermée Fiasp plus pramlintide réalisées avec des patients vivant dans un cadre ambulatoire sont justifiées », ajoute le Dr Tsoukas.
Pour sa part, Ahmad Haidar conclut en disant : « Les conclusions tirées dans l’étude que nous venons de réaliser constituent une percée en vue de la mise au point d’un système d’administration d’insuline entièrement automatisé, qui allège le lourd fardeau accablant les personnes vivant avec le diabète de type 1, sans pour autant dégrader le contrôle de la glycémie. Cette percée peut avoir des conséquences importantes pour les quelque 300 000 Canadiens vivant actuellement avec le diabète de type 1, sans compter les nombreuses autres personnes se trouvant dans une situation similaire dans le monde entier. »
À propos de l’étude
Lire la publication dans Lancet Digital Health
Les auteurs tiennent à souligner le soutien indéfectible qu’ils ont reçu de la part de Diabète Canada et à exprimer leur profonde reconnaissance aux participants à l’étude.
En savoir plus en consultant le site Web de l’Université McGill
11 novembre 2021